 By Andrew Alexander, MSc Student at Strynadka Lab, CBR
By Andrew Alexander, MSc Student at Strynadka Lab, CBR
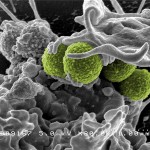
Interaction of MRSA (green bacteria) with a human white cell. MRSA is a leading cause of hospital-associated infections in the United States and United Kingdom. Credit: NIAID
Antibiotic resistance: a troubling reality that has captured the attention of medical doctors, scientists, and patients alike. The ability of certain bacteria to grow in the presence of antibiotics at concentrations that would normally be lethal is an extremely pervasive problem that is interfering with our ability to treat some bacterial infections. In 1999 a team of researchers from the Lewin Group showed that patients infected with methicillin resistant Staphylococcus aureus had nearly 3 times the mortality rate compared to patients infected with a responsive strain. In fact, infections with antibiotic resistant bacteria are not rare, isolated incidences. According to a report produced by the Centre for Disease Control and Prevention (CDC) in 2013, there are over 2 million infections with resistant bacteria and more than 23,000 directly attributable deaths each year in the US alone.
This is not a new problem, but rather one that has been multiplying ever since the first antibiotics came into use. In 1928, Sir Alexander Fleming noted that fungus contaminating his petri dishes inhibited the growth of some bacteria, leading to the discovery of penicillin. Shortly thereafter, it was also discovered that bacteria could become resistant to penicillin if grown in non-lethal concentrations of the drug, as noted in his Nobel lecture. We now know that sub-lethal concentrations of antibiotic can cause an accumulation of mutations in the bacterial DNA sequence, allowing the bacteria to cope with the drug’s actions. As beneficial mutations spread through the culture via natural selection, antibiotic resistance can rapidly disseminate through a population of bacterial species.
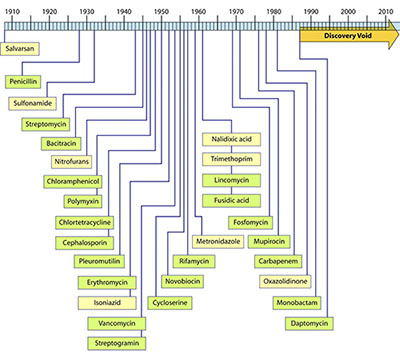
Timeline of the discovery of antibiotic classes showing the dearth of progress made in the last few decades (Silver, L. L. Clin Microbio Reviews, 2011)
In an excellent review Origins and Evolution of Antibiotic Resistance, Davies & Davies note that resistant infections were seen in patients only a few years after the first synthetic derivatives of penicillin were released onto the ailing. The growing deluge of antibiotic resistance was partially avoided with a remarkable string of novel antibiotics that were initially effective against some pathogenic bacteria. However, as shown in the figure, over the last few decades significantly fewer new classes of antibiotics have come down the research and development pipelines. This decrease has been attributed to a variety of causes ranging from companies being reluctant to invest in this area to the repeated screening of a small, relatively homogenous population of bacteria for the production of antibiotics. Recently, Liu and Pop outlined over 6200 significantly different proteins involved in bacterial resistance, demonstrating the complexity of the mechanisms involved.
As the issue of antibacterial resistance becomes more widespread and mainstream, experts – and increasingly the public – want to know the answers to questions such as: What is the extent of the problem? Why aren’t we producing new classes of antibiotics that will tackle the resistance problem? Why does resistance continue to be such a great problem? How are bacteria becoming resistant on a molecular level? The lack of answers to these questions prompted the World Health Organization (WHO) to release “Antimicrobial resistance: Global report on surveillance” in 2014- the first world-wide report on antimicrobial resistance. This report demonstrates that we may reach the post-antibiotic era this century unless we can take action on many different fronts. Progress on this issue will require input and change from many areas of society, ranging from alterations in government policy regarding the distribution of antibiotics and the allocation of funds for research into this area, to changing how antibiotics are prescribed and addressing issues of patient compliance through education. However, this field of study cannot progress without reliable funding of basic research into resistance at a fundamental level.
This fundamental research into the workings of antibiotic resistance is distinct from applied or industrial research, which typically involves projects that have a clearly delineated goal that will produce a marketable item within a short time frame. While applied research is vital for optimizing novel ideas for the real world, it is poor at contributing breakthroughs in science. Basic research is essential to determine how current drugs can be modified to perhaps restore sensitivity to antibiotics or investigate pathways in bacterial metabolism that could be promising targets for newly designed drugs, allowing us to discover potential inhibitors.
At the Centre for Blood Research, the Strynadka lab is dedicated to understanding bacterial antibiotic resistance mechanisms and how they can be inhibited at the most fundamental level, that of molecular interactions. Current projects involve analysing molecular interactions to understand the essential bacterial processes required for growth and elucidating the mode of action for new antibiotics. “Seeing” how molecules interact can allow us to design better antibiotics and to predict how resistance might arise in the future.


