By Brianne Burkinshaw, PhD Candidate at Strynadka Lab, CBR
 In a recent issue of Structure, Matthew Solomonson, a graduate student in the Strynadka Lab at the Centre for Blood Research, and colleagues describe the structure of a virulence factor called EspB from Mycobacterium tuberculosis (Mtb), a pathogen that causes tuberculosis (TB). Mtb has an unusually dense cell wall that likely protects Mtb from the host’s immune system. However, at the same time, it creates a challenge for transportation of proteins into the environment. In order to secrete virulence factors across the cell wall, Mtb uses a specialized protein transport system called ESX-1 Type VII Secretion System (Schematic overview, below). Many of these secreted virulence factors remain poorly characterized. In the current paper, Solomonson and colleagues solved the structure of the ESX-1 secreted virulence factor EspB, demonstrating that it has a pore-like structure, which may facilitate its proposed membranolytic function during pathogenesis.
In a recent issue of Structure, Matthew Solomonson, a graduate student in the Strynadka Lab at the Centre for Blood Research, and colleagues describe the structure of a virulence factor called EspB from Mycobacterium tuberculosis (Mtb), a pathogen that causes tuberculosis (TB). Mtb has an unusually dense cell wall that likely protects Mtb from the host’s immune system. However, at the same time, it creates a challenge for transportation of proteins into the environment. In order to secrete virulence factors across the cell wall, Mtb uses a specialized protein transport system called ESX-1 Type VII Secretion System (Schematic overview, below). Many of these secreted virulence factors remain poorly characterized. In the current paper, Solomonson and colleagues solved the structure of the ESX-1 secreted virulence factor EspB, demonstrating that it has a pore-like structure, which may facilitate its proposed membranolytic function during pathogenesis.
EspB Forms a Heptameric Ring
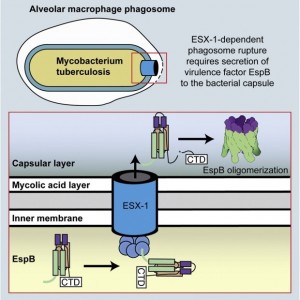
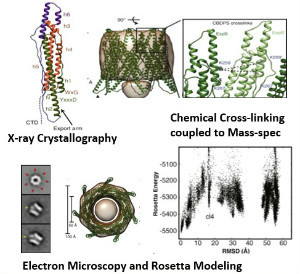
To gain insight into the structure and activity of EspB, Solomonson et al. solved the X-ray crystal structure of the monomeric EspB. They discovered a conserved secretion signal that allows it to be recognized by the ESX-1 system. Additionally, they observe that EspB forms a complex in solution. The authors used a combination of electron microscopy, cross-linking and Rosetta modeling to build a model of the complex, discovering that seven units of EspB are necessary for the assembly. This heptamer is organized into a pore-like structure, with the hydrophobic helical tip of each monomer at one end of the complex, and a C-terminal domain that is processed by an ESX-1 protease at the opposite end of the pore. The overall structure of the EspB complex supports a membrane puncturing function.
Solomonson reflects on the larger implications of understanding the structure of ESX-1 system virulence factors:
“The thing I’m most excited about is that if we improve our understanding of the ESX-1 system, scientists can potentially improve anti-TB vaccines. People are interested in ESX-1 because it is absent from the current vaccine strain, which was developed nearly a century ago. Imagine introducing ESX-1 into this vaccine strain: the immune system would be able to “see” the key secreted antigens like EspB and can adapt and learn to reduce their harmful effects. It would be like taking the venom out of a snake so that you can learn to fight snakes safely, and when the real venomous snake comes along, the body can take care of itself.”