
 Written by Emily Chan, CBR-SBME Summer Student alum, Strynadka Lab (left)
Written by Emily Chan, CBR-SBME Summer Student alum, Strynadka Lab (left)
Edited by Bronwyn Lyons, PhD Candidate, Strynadka Lab (right)
Antibiotics are used to treat bacterial infections by killing or inhibiting the growth of bacteria1. However, bacteria can develop resistance to antibiotics, which each year accounts for at least 700,000 deaths worldwide2. This is becoming an increasingly dangerous threat to society as resistance continues to rise globally1. To prevent entering a post-antibiotic era, there have been extensive attempts to develop novel antibiotic treatments3.
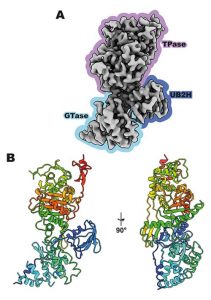
Figure 1: Structure of PBP1b from E. coli determined by cryo-EM3. A) The structure of apo PBP1b at 3.28 Å resolution with the indicated domains: the peptide-linking (TPase) domain in purple, the putative activator binding (UB2H) domain in indigo, and the sugar-linking (GTase) domain in blue. B) A cartoon representation of apo PBP1b with rainbow colouring from N to C terminus (blue to red).
After returning from summer break in 1928, Alexander Fleming found a strange mold growing on one of his Staphylococcus petri dishes4. Interestingly, the area around this mold showed no bacterial growth. His finding that the secretions from this mold were able to kill bacteria lead to the discovery of the first antibiotic: penicillin. Howard Florey and Ernst Chain continued Fleming’s work to turn penicillin into the life-saving drug that we know. β-lactam antibiotics, such as penicillin, kill bacteria by preventing synthesis of the bacterial cell wall, which is made up of sugar chains cross-linked by peptides to provide protection and support to the bacterial cell. β-lactams inhibit cell wall synthesis by binding and inhibiting PBPs localized in the membrane. In a recent publication by Caveney, et al., the antibiotic-free structure of a penicillin-binding protein from the human pathogen Escherichia coli (PBP1b) was determined using single-particle cryogenic electron microscopy (cryoEM) in a detergent-free system using styrene maleic acid (SMA) (Figure 1) 3,5. PBP1b belongs to the class A PBPs which can perform both a sugar-linking and peptide-linking activity. This paper describes the first structure of a PBP in a detergent-free system, which provides a more representative structure of native PBP1b in comparison to detergent extracted PBP1b.
Previously, the molecular structures for antibiotic-bound PBP from E. coli were described to contain three distinct domains: 1) the sugar-linking domain, 2) the putative activator binding domain, and 3) the peptide-linking domain. The peptide-linking domain, known as the transpeptidase, is inhibited by β-lactam antibiotics, preventing the cross-linking of cell wall sugar chains with peptides, resulting in weakening of the cell wall. Unfortunately, many harmful bacteria are developing resistance against β-lactams by producing β-lactamases – enzymes that break down these antibiotics. As well, much of the information on the critical active site features of PBPs are still unknown3. Having a better understanding of the structure of PBPs is the first step to developing and optimizing antibiotic treatments against harmful bacteria.
The structure presented by Cavaney and colleagues represents the first study of a PBP employing cryoEM, as well as the detergent-free system SMA. In their structure, they observed that the interface between the three domains of PBP1b were complex and dynamic. In comparison to previously described antibiotic-bound structures of PBP1b, they found that their structure for the antibiotic-free form of PBP1b had a distinct conformation; with an approximate 40° rotation difference of the sugar-linking domain in reference to the peptide-linking domain. They suggest that this rotation is likely induced by the binding of PBP1b to an antibiotic, justifying the importance for understanding the antibiotic-free structure for the future development and optimization of PBP-specific antibiotics.
The structures for class A PBPs have been elucidated for only five different pathogens and not much is known about their structure3. This work describes the potential of using cryoEM to better determine the structure of class A PBPs, such as PBP1b3. Cryo-EM also unlocks the potential to determine the structure of class A PBPs from other species to further aid efforts toward drug development against infectious bacteria.
References
1 WHO. Antibiotic resistance. Published 2020. Accessed July 9, 2021. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
2 O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Published online 2016.
3 Caveney NA, Workman SD, Yan R, Atkinson CE, Yu Z, Strynadka NCJ. CryoEM structure of the antibacterial target PBP1b at 3.3 Å resolution. Nat Commun. 2021;12(1):1-7. doi:10.1038/s41467-021-23063-6
4 Discovery and Development of Penicillin. American Chemical Society International Historic Chemical Landmarks. Accessed August 14, 2021. https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/flemingpenicillin.html
5 Sun C, Gennis RB. Single-particle cryo-EM studies of transmembrane proteins in SMA copolymer nanodiscs. Chem Phys Lipids. 2019;221:114-119. doi:10.1016/j.chemphyslip.2019.03.007


