During the normal healing process, fibrous connective tissue forms at the site of injury or damage. However, in some cases, deregulation of this reparative process leads to excess tissue deposition, called fibrosis. It interferes with the function of the underlying tissue and can cause a wide range of diseases, such as liver cirrhosis and pulmonary fibrosis to name a few. Tissue fibrosis diseases cause nearly 45% of all deaths in industrialized nations and currently no efficient antifibrotic therapeutic interventions exist.
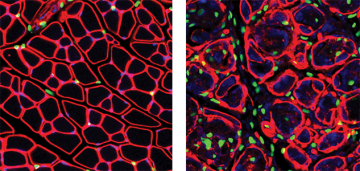
The Rossi laboratory, a member of the CBR and the Biomedical Research Centre, identified a population of multipotent connective tissue progenitor cells, called fibro/adipogenic progenitors (FAPs) that proliferates in response to muscle damage. Normally, in healthy muscle, FAPs expand to generate transient connective cells to support muscle function, followed by rapid reduction in their numbers. However, in conditions favoring degeneration, FAPs persist in the tissue and differentiate into collagen-producing fibroblasts and adipocytes, which proceed to infiltrate the muscular tissue, causing fibrosis.
Now, Dr. Rossi’s team unraveled one of the mechanisms governing FAP fate, published in Nature Medicine on June 8, 2015. During the normal healing process pro-inflammatory macrophages directly induce cell death, or apoptosis, in FAPs through expressing tumor necrosis factor (Tnf), which prevents their differentiation into matrix-producing cells. In a mouse model of chronic muscle injury (the mdx mouse model of muscular dystrophy), the Rossi lab found that most macrophages express both Tnf and Tgf-β1 simultaneously, and that TGFb efficiently blocks Tnf, thereby failing to induce FAP death and clearance leading to collagen deposition and fibrosis. They further showed that Nilotinib, a clinical tyrosine kinase inhibitor with known anti-fibrotic effect, blocks Tgf-β1–activated pathways in these cells and restores FAP apoptosis.
This latest discovery strongly suggests that the interaction between FAPs and Macrophages could be a target of the new anti-fibrosis therapies.
This study was highlighted in UBC News.