In mammalian cells, signaling by Notch proteins is a powerful way to influence gene regulation. This pathway most commonly governs cell differentiation, which may be activated or suppressed by Notch depending on the target cell identity. In such cases, Notch pathway signalling activates genes that supress differentiation, instead allowing the target cell to proliferate. As a promoter of cell proliferation, deregulation in the Notch pathway is a powerful indicator of the cancerous tumor progression — otherwise known as tumorigenesis. Currently, drug development targeting this pathway provides great therapeutic potential against the spread of cancer. Notch does not bind DNA directly, but rather binds to a repressor, RBPJ, which binds DNA. Binding of RBPJ by Notch results in release of transcriptional repressor proteins from RBPJ. This event recruits transcriptional activators, resulting in derepression and/or activation of various downstream targets. By examining the impact of RBPJ loss, CBR member Dr. Aly Karsan, and his research group unveiled a new oncogenetic pathway that may be active in various cancer types, published in the December 2014 issue of the Journal of Experimental Medicine, viewed here.
The group demonstrated that loss of RBPJ mimics a Notch activation signal through the derepression of a cohort of genes that are also transcribed following Notch activation. Previous studies have illustrated that in tissue from more aggressive breast cancers, RBPJ is often depleted. Karsan et al. found that similarly to faulty Notch signalling, the depletion of RBPJ yields enhanced tumorigenicity in cancerous mouse models. Using RBPJ-depleted or null tumor cells (RBPJ -/-), implanted tumors were protected from cell death, enabling enhanced tumor progression in xenograft models.This effect was intensified in more hypoxic areas of the tissue. The study proposed that the observed increased cell survival may be carried out through MYC and NF-ƙB signalling, which was enabled in RBPJ-depleted cells. Interestingly, while the genetic signature resulting from RBPJ loss is similar to that seen with aberrant Notch activity, the activation itself works independently from Notch protein activation. In essence, RBPJ depletion provides an alternate stimulus in signalling tumorigenesis.
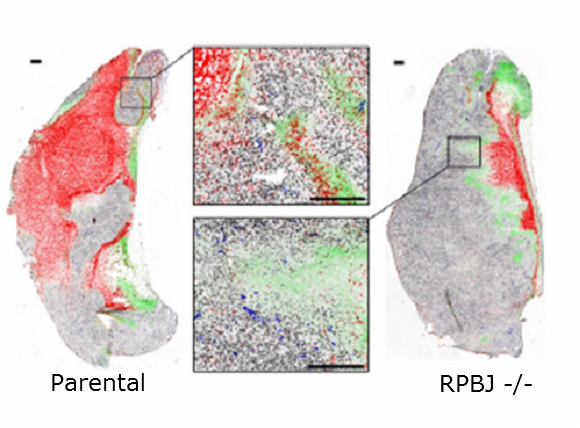
Increased cell survival of RBPJ depleted (right) tumor cryosections after the induction of cell death. Dead cells are stained red, proliferating cells are black and hypoxic areas are stained green.
While the study focused exclusively on breast and lymphoma model systems, analysis of clinical evidence independent from the Karsan lab suggests RBPJ depletion may also play a role in other malignancies. The discovery of an alternate tumorigenesis activator sheds new light on the treatment of more aggressive cancers. Inhibitor development against the signals resulting from RBPJ depletion (i.e MYC and NF-ƙB ) may be beneficial in circumstances where Notch inhibitors prove ineffective.
This research was supported by grants to A. Karsan from the Canadian Institutes of Health Research (CIHR), the Cancer Research Society and Studentships from the Michael Smith Foundation for Health Research.
Article Contributed by Erika Siren, PhD Student at Kizhakkedathu Lab, CBR


