 By Dustin King, PhD Candidate in Strynadka Lab, CBR
By Dustin King, PhD Candidate in Strynadka Lab, CBR
Uncontrolled blood loss is the most common cause of death in severe trauma, which cumulatively results in more than five million deaths per year. Similarly, post-partum hemorrhage is the most common cause of maternal mortality. Among these patients, uncontrolled bleeding is the leading cause of preventable death. In open wounds, bleeding can be controlled, in part, by delivering therapeutics topically to the damaged blood vessels. However, getting therapeutics to the affected vasculature presents a major drug delivery challenge, largely because these agents must be transported against the blood flow to the actual source of the bleed, which is not always known with precision.
To address this challenge of drug delivery in uncontrolled bleeding, James Baylis, a PhD student in Dr. Christian Kastrup lab at the CBR, spearheaded a collaborative study that was recently published in the journal Science Advances characterizing a self-propelled drug delivery system capable of penetrating deep into the wound site.
Schematic showing CaCO3 particles releasing CO2 gas and transporting themselves and molecular cargo through solution. Source: Ronsmans and Graham (2006). The Lancet.
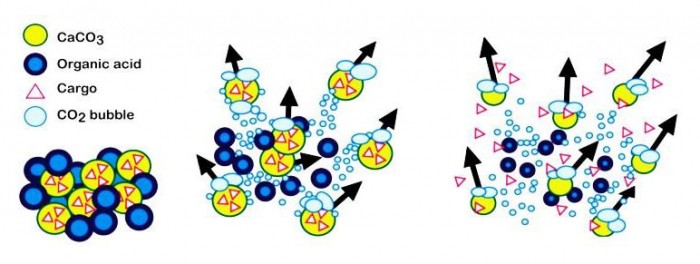
Baylis and colleagues were able to use gas-generating microparticles that consisted of a mixture of calcium carbonate (CaCO3) and tranexamic acid – materials that are cheap and readily available. When this mixture is injected into blood, the particles react vigorously to produce CO2 gas, much like an Alka-Seltzer dropped in water. This reaction causes the particles to rise buoyantly, propel laterally, and move due to convective forces generated by the CO2 gas bubbles. In mice, these forces also help the particles carry cargo much further into the vasculature than non-propelling control particles.
Experiments performed in microfluidic devices suggest that these forces also enable the particles to carry cargo against flowing whole blood. Based on these experiments, the team concluded that CaCO3 particles are capable of propelling cargo against the flow velocities typically present in wound sites and small vessels.
The ability of these microparticles to transport molecular cargos through blood could address an unmet need for delivering therapeutic materials to halt severe bleeding. For example, in the case of severe bleeding, they can be used to deliver thrombin, a clotting factor that has many documented roles in hemostasis. To create self-propelling particles that clot blood, thrombin was adsorbed onto the CaCO3delivery system. When loaded with thrombin and administered topically, the particles prevented severe hemorrhage in three in vivo models of surgery-induced traumatic bleeding. Baylis used the participles to stop bleeding in a mouse tail amputation experiment, a mouse liver puncture, and arterial hemorrhage in a pig.
Taken together, this study represents an important initial proof of concept that relatively inexpensive self-fueling microparticles can enhance drug delivery in live animals. However, the authors are careful to point out that additional studies are required to prudently assess safety and toxicity for various medical applications. When asked about the therapeutic potential of these particles Baylis concludes that: “following rigorous preclinical safety studies, these particles have the potential to stop bleeding and prevent death in a wide range of clinical scenarios, including during childbirth, surgery and following major trauma”. Although this work focused on transporting coagulants, many other medical applications have been suggested for self-fuelled drug delivery systems. This technology is just in its infancy and the true breadth of potential medical application is yet to be realized.




