 By Dr. Stefanie Novakowski, Kastrup Lab
By Dr. Stefanie Novakowski, Kastrup Lab
Originally posted on the Canadian Blood Services R.E.D. blog with permission.
The ability to genetically modify a cell is a powerful tool. Genetically modified cells have advanced our understanding of how the body works and how diseases develop. They are currently used as treatments for a range of diseases, from cancers to bleeding disorders. Yet not all cells are easily modified. This is true of small cells that are indispensable for stopping blood flow during injury.
Why modify platelets? Platelets are currently used in the clinic to stop ongoing bleeding, caused by trauma or surgery. However, during severe bleeding platelets become dysfunctional, and these platelet transfusions no longer work. Correcting platelet dysfunction during trauma could be addressed by modifying platelets. For example, platelets could be engineered to produce proteins that activate clotting or stabilize blood clots after they form. Platelets are also given to patients with low platelet counts, which can be caused by a variety of factors, including certain cancers. While the platelets are given to patients to prevent bleeding, platelets also naturally interact and communicate with cancer cells. Modified platelets have the potential to deliver cancer-fighting materials to tumour cells, while also preventing bleeding.
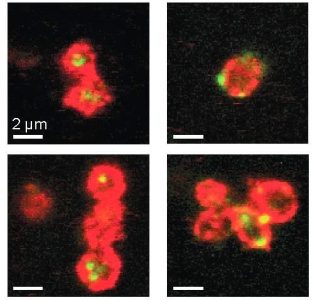
Microscope images of platelets (red) that have taken up foreign genetic material (green). Adapted from: Novakowski et al: Delivery of mRNA to platelets using lipid nanoparticles. Scientific Reports 2019: 9; 552.
Unfortunately, commonly used methods for delivering genetic material to platelets either don’t work well or induce unwanted changes to the platelets. Currently, the best method for creating modified platelets is to modify the cells they develop from, called hemopoietic stem cells. These stem cells can be grown inside of a laboratory, modified, and then transplanted back into an individual. This approach has been used to treat inherited diseases. However, this method permanently changes a person’s platelets, and would not be useful in scenarios where only temporary modifications to the platelets are required, such as during active bleeding. Platelet BioGenesis, a company based in the United States, has recently developed a technology for growing large numbers of platelets from modified hemopoietic stem cells, but it will be years these laboratory-grown platelets can be used in hospitals.
To address this problem, the Kastrup Laboratory at the University of British Columbia is taking a novel approach to directly modifying platelets. Using small, synthetic spheres called lipid nanoparticles, we successfully delivered genetic material into platelets without inducing unwanted changes to the platelets. By using specific lipids, or fats, to build the nanoparticles, we identified nanoparticles that deliver messenger RNA to the platelets. Like DNA, messenger RNA is a type of genetic material that is read by a cell to create new proteins. Unlike most cells, platelets do not have their own DNA, so genetically modifying platelets requires delivery of messenger RNA.
By testing various classes of these nanoparticles, we determined characteristics of the nanoparticles that are important for uptake. These characteristics included a small size for the particle, and positive charge on its surface. Platelets treated with nanoparticles still had the ability to aggregate and spread, processes that are important to their ability to stop blood flow. We also found that the RNA taken up by the platelets was then released back out under certain conditions, indicating that modified platelets have the potential to be used a delivery vehicle for RNA-based drugs. This might be useful for creating cancer-fighting platelets, as RNA that promotes cancer cell death could be delivered to tumours by the RNA-treated platelets.
Delivery of RNA to the platelets is only the first step in creating genetically modified platelets. To modify a platelet, the RNA has to be read by the cell, creating a new protein that alters the platelet’s function. Unfortunately, the RNA that we delivered could not be read by the platelets. To address this, we are now varying the composition of the nanoparticles, using different types of lipids to build the particles. This may alter where the RNA is delivered within the platelet and allow the RNA to be read by the platelets. Further experiments are also needed to see whether RNA-treated platelets can function once they are placed back into a patient. Currently, the platelets are modified after they have been isolated from whole blood from healthy individuals, and their function has only been assessed in a test tube. Whether these platelets still circulate and respond to signals within circulating blood remains to be seen.
Platelets are involved in bleeding, cancer, and even immune and inflammatory disorders. The ability to directly modify platelets would extend our knowledge of platelet biology, and hopefully lead to improved therapeutics for a range of diseases. Platelet transfusions are already essential to treating bleeding. If researchers are successful in creating modified platelets that can be used in hospitals, there may be even more opportunities for platelets to act as a life-saving therapy in Canada and the throughout entire world.
In January 2019, Dr. Stefanie Novakowski completed her PhD at the University of British Columbia in the laboratory of Dr. Christian Kastrup. There, she developed new tools for modifying platelets – small cells found within the blood that are responsible for blood clotting. As a graduate student, Stefanie discovered a passion for knowledge translation, and the Canadian Blood Service’s inaugural writing competition provided the perfect opportunity for her to hone her writing skills. Stefanie’s own work provided the perfect inspiration for her writing, since it was a story she knew but rarely shared outside of academia. Now that she has completed her degree, Stefanie hopes to start a career in science communication.
The 2018 Canadian Blood Services Lay Science Writing Competition was organized by the Canadian Blood Services’ Centre for Innovation with welcome support from Science Borealis and the Centre for Blood Research at the University of British Columbia.


