The dynamic and tightly regulated interactions of actin and actin-binding proteins determine the sape and function of all mammalian cells. Loss of integrity of these proteins and their capacity to modulate cell function under different pathophysiologic conditions are associated with a range of diseases. It is therefore critical to delineate the mechanisms underlying these interactions in health and disease. Using X-ray crystallography and computer-generated simulations, CBR investigator Les Burtnick and his PhD student Hui Wang, in collaboration with scientists in Singapore, are doing just that!
Actin is among the most abundant of animal cell proteins. Through dynamic participation in a meshwork of proteins called the cytoskeleton, actin filaments determine the shapes and functions of essentially all cells. For example, rapid and controlled disassembly and reassembly of the cytoskeletal system of white cells is needed for them to be able to chase down and engulf bacteria and other invading organisms, while functionally intact actin filaments in cardiomyocytes are required for the heart to be able to pump blood.
Gelsolin is the founding member of a family of actin-binding proteins that includes, among others, villin. These intracellular proteins modulate actin filament dynamics through a range of distinct activities that include actin filament severing, capping, bundling, nucleation and elongation. Priming of these activities however, requires that the normally tightly folded regulatory protein package be opened up. The energy needed to drive this transformation comes from multiple sources, including attractive electrostatic forces between charged calcium ions and opposite charges on gelsolin-like proteins.
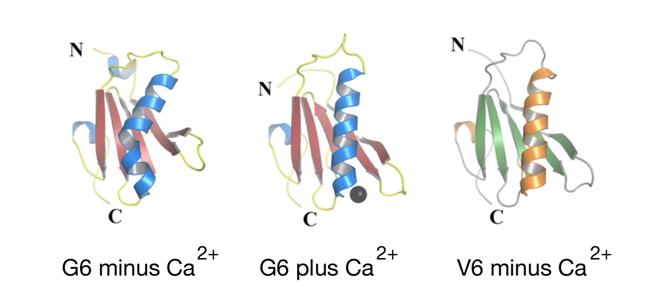
CBR PhD student Hui Wang used X-ray crystallography, coupled with computer-generated simulations, to model activation of gelsolin and villin in the context of calcium and actin. Hui, together with supervisor Les Burtnick of the CBR and collaborators in the Bob Robinson and Ivana Mihalek laboratories in Singapore, discovered that the long helix that forms the backbone of the calcium-free structure of the isolated V6 domain of villin is elongated, and closely matches that in the activated calcium-bound G6 domain of gelsolin. This conformation is in striking contrast to the kinked conformation of the long helix in intact inactive G6 found under calcium-free conditions.
From these observations, the authors deduced a novel and intriguing paradigm that explains how the energy for activation of gelsolin may be partly derived by calcium-induced spring-like straightening of protein helices from their distorted inactive forms, thereby opening “latches” to expose otherwise buried surfaces that can bind actin and regulate its function. Hui et al are thus advancing our understanding of the fundamental mechanisms by which the cytoskeleton controls numerous key cellular processes – information that the authors hope will result in diagnostic and therapeutic breakthroughs.
Get the whole story in JBC (2009) 284, 21265 – 21269


