By Preety Panwar, Postdoctoral Fellow at Bromme lab, CBR
Collagen is the most abundant protein in the human body and constitutes up to 90 % of the organic material in bones and is a significant component of skin, arterial walls, and ligaments. It provides structural and mechanical support to tissues and its excessive degradation can lead to osteoporosis, arthritis, aneurysms, and skin aging. In a recent study published in PNAS, Bromme lab at the Centre for Blood Research describes the mechanism by which collagen fibers get degraded by cathepsin K.
Besides matrix metalloproteases, cathepsin K is the major collagen enzyme responsible for osteoclast mediated collagen breakdown in bones. Therefore, it also represents a key pharmaceutical target for the treatment of osteoporosis. However it was unknown how cathepsin K breaks down the tightly packed collagen fibers. In this study, we have shown that cathepsin K forms dimers, which are needed for the partial unfolding of the triple-helical collagen molecules prior to their proteolytic cleavage. Our previous research demonstrated that glycosaminoglycans (GAG), such as chondroitin sulfate, are required for this process. Here we furthered that idea by showing that cathepsin K dimers specifically bind to the surface of collagen fibers via the glycosaminoglycan molecules. These cathepsin-K/GAG complexes then cause the release of soluble tropocollagen fragments, leading to the dissolution of the fibers.
This study suggests that interfering with the dimerization of the protease or its binding to glycosaminoglycans would selectively abort the activity of the protease without interfering with its other proteolytic function. Therefore, this research not only provides understanding of how cathepsin K breaks down collagen fibers but also opens the door for the design of a new class of drugs selectively targeting the degradation of collagen through cathepsin K.
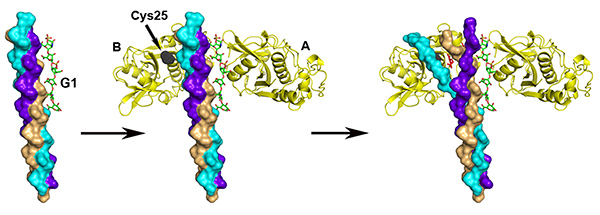
Schematic representation of the proposed mechanism of collagen degradation by CatK dimers. CatK dimer binds to a collagen–GAG complex which is unfolded before being cleaved by the active site (Cys25).


