 By Deb Chen, PhD Candidate at Devine Lab, CBR
By Deb Chen, PhD Candidate at Devine Lab, CBR
The discovery of antibiotics transformed patient care and dramatically advanced medical interventions and treatments. However, the relentless rise of antibiotic resistance over the years continues to threaten the diverse fields of medicine, including surgery, cancer chemotherapy, transplantation medicine and others.
Recent PhD graduate, Dr. Dustin King, from the Strynadka Lab at the CBR, discovered unexpected properties of the newly FDA-approved antibiotic enhancer, avibactam. The drug was expected to block the bacterial defenses against broad spectrum antibiotics. However, it was also found to have direct anti-microbial properties, making it the first such drug introduced to the market within the last 30 years.
Bacterial cells are unique from ours in that they have an essential cell wall structure, which is constructed of a massive net-like ‘peptidoglycan’ protective layer that envelops the entire cell. The synthesis of peptidoglycan involves penicillin-binding proteins, or PBPs, which were characterized through their affinity for penicillin.
Penicillin, cephalosporins, and other related antibiotics share a similar core structure, the β-lactam ring, and thus are collectively referred to as β-lactam antibiotics. These are the most common types of antibiotics used in the clinics, for their potent ability to kill a broad range of bacteria. b-Lactam antibiotics are able to bind to the PBP active site and irreversibly inactivate their function, which then interrupts the formation of the peptidoglycan layer. This subsequently leads to irregularities in the bacterial cell wall structure (e.g., elongation defects) and results in cell death.
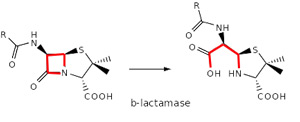 Over time, however, bacteria acquired the ability to produce protective enzymes, such as b-lactamases, which are able to break the b-lactam ring open and deactivate the drug’s antibacterial properties.
Over time, however, bacteria acquired the ability to produce protective enzymes, such as b-lactamases, which are able to break the b-lactam ring open and deactivate the drug’s antibacterial properties.
Published in ACS Infectious Diseases, Dr. King and the research team in the Strynadka lab at the CBR, found that small amounts of avibactam and other diazabicyclooctane (DBO) derivatives were effective in inhibiting the growth of different multi-drug resistant Gram-negative clinical pathogens. This ability was independent of the presence of protective bacterial b-lactamases, suggesting that these drugs can act as direct antimicrobial agents. They further demonstrated that these DBO derivatives, in addition to protecting against b-lactamases, also directly act on PBP to inhibit the synthesis of the peptidoglycan layer and to disrupt cell wall structure.
“The DBO b-lactamase inhibitor constitutes the only such drug to be introduced into the clinic in over 30 years,” commented Dr. King. He is hopeful to play a part in the ongoing world-wide battle to “revive our current b-lactam [antibiotics] pool” through the development of novel b-lactamase inhibitor compounds. This finding is pivotal in the global effort to ensure public health and in finding new tools to design next generation pharmaceuticals with expanded spectra of activity against currently untreatable, antibiotic resistant pathogens.


