
 Written by: Stephanie Besoiu & Clara Xia – PhD Students, Jefferies Lab
Written by: Stephanie Besoiu & Clara Xia – PhD Students, Jefferies Lab
Edited by: Alexandra Witt, PhD Candidate, Pryzdial Lab
Approximately 2 in 5 Canadians will be diagnosed with cancer in their lifetime, and yet we still have a limited understanding of cancer immunity, greatly impacting treatment efficacy (1). One underexplored area is the research into which immune cells help prime and carry out anti-tumour immune responses.
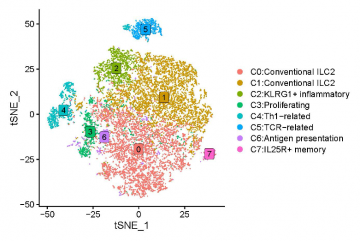
Figure 1: Graph-based clustering of ILC2s based on their transcriptional profiles, identifying eight subpopulations of ILC2s. Some specific populations may have a potential role in antitumor immune responses. Each dot represents a single cell.
In the latest study by the Jefferies lab, we delve into the intricate world of type-2 innate lymphoid cells (ILC2), specifically exploring their plasticity during tumour expansion (2). ILC2s are a recently discovered type of immune cell, conventionally involved in maintaining tissue homeostasis and responding to various environmental challenges such as parasite infection and allergic disease (3,4). The general involvement of ILC2s in the immune response to various tumours has been studied, however their specific functions in different tumour types remain controversial. Some evidence implicates ILC2s in the promotion of tumour development, while our lab’s earlier research established that ILC2s play a crucial role in supporting anti-tumour Th1 responses by promoting interactions between the innate and adaptive components of the immune system. Additionally, mounting evidence points to a novel function for ILC2 in activating cytolytic T lymphocytes, which aids in the reduction of tumour growth and metastasis (5,6). To explain these contradictory findings, we proposed that there may be multiple undescribed subpopulations of ILC2s. To address this, we used single-cell RNA sequencing on lung-derived ILC2s from tumour-bearing mice, and with or without external ILC2 activation by the cytokine IL-33. With the single-cell RNA sequencing approach, we were able to analyze individual cells within a population, obtaining much greater detail on the genetic and transcriptional heterogeneity that can be masked in bulk RNA sequencing methods. Additionally, single-cell sequencing enabled the identification and characterization of rare cell types that might be present in low abundance within a sample.
The results of the study revealed a surprising diversity of novel ILC2 subpopulations. These subpopulations exhibited distinct genotypes which infer different functional characteristics, suggesting specialized roles in the tumour microenvironment. For example, we found populations marked for different phases of cell development: proliferation, transition, terminal, and memory. The main portion of the ILC2s retained conventional type-2 identity while being able to acquire heightened T-cell recruitment and type-1 signatures (C0 and C1) during tumour development (see Figure 1). Other smaller subpopulations showed signatures for antigen-presentation (C6), KLRG1+ inflammatory (C2), IL25R+ memory (C7), and Th1-related (C4) functions, the latter of which may play an important role in effective anti-tumour responses. Interestingly, these clusters changed under different conditions, shifting towards beneficial pro-inflammatory immunity during both tumour development and external IL-33 activation. The identification of these previously unrecognized ILC2 subsets challenges traditional views and underscores the importance of ILC2s in immune surveillance.
This paper advances our understanding of the role of ILC2s in the context of tumour growth. We discovered and characterized several new ILC2 subpopulations, providing valuable insights into the specific mechanism and functions of ILC2s in the tumour microenvironment. By deciphering the crosstalk between ILC2 and neighbouring immune cells and tumour cells, we aim to further elucidate the broader impact of ILC2 diversity on the overall anti-tumour immune responses and pave the way for innovative and effective therapeutic strategies in cancer treatment.
Link to article: https://www.nature.com/articles/s42003-023-05536-0
References
- Cancer statistics | Canadian Cancer Society [Internet]. [cited 2024 Jan 30]. Available from: https://cancer.ca/en/research/cancer-statistics
- Xia CW, Saranchova I, Finkel PL, Besoiu S, Munro L, Pfeifer CG, et al. A diversity of novel type-2 innate lymphoid cell subpopulations revealed during tumour expansion. Commun Biol. 2024 Jan 3;7(1):1–15.
- Mathä L, Martinez-Gonzalez I, Steer CA, Takei F. The Fate of Activated Group 2 Innate Lymphoid Cells. Front Immunol. 2021;12:671966.
- Kim BS, Artis D. Group 2 Innate Lymphoid Cells in Health and Disease. Cold Spring Harb Perspect Biol. 2015 May;7(5):a016337.
- Wu L, Zhao W, Tang S, Chen R, Ji M, Yang X. Role of ILC2s in Solid Tumors: Facilitate or Inhibit? Frontiers in Immunology [Internet]. 2022 [cited 2024 Jan 30];13. Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2022.886045
- Saranchova I, Han J, Zaman R, Arora H, Huang H, Fenninger F, et al. Type 2 Innate Lymphocytes Actuate Immunity Against Tumours and Limit Cancer Metastasis. Sci Rep. 2018 Feb 13;8:2924.


