 By Sreeparna Vappala, Graduate Student, Kizhakkedathu Lab
By Sreeparna Vappala, Graduate Student, Kizhakkedathu Lab
Historically, heparins were the mainstay of anticoagulant therapy. They were the primary agents used to treat deep vein thrombosis, arterial thromboembolism, and ischemic stroke. In spite of the introduction of anti-Xa and anti-thrombin agents to treat/prevent thrombosis, heparin continues to be used in major surgeries like cardio-pulmonary bypass to prevent blood clotting. Although heparins are effective in preventing blood clots, the risk of bleeding associated with their use is a major clinical concern. Hence, it is important to be able to neutralize heparins. The only FDA approved heparin antidote is protamine sulphate (protamine). Protamine is a positively charged peptide that binds to negatively charged heparin via electrostatic interactions. However, protamine has many limitations. Firstly, it has only a small range of doses where it can effectively neutralize heparins. At higher doses, protamine binds non-specifically to blood proteins and shows some anticoagulant effects. Secondly, protamine is only able to completely neutralize unfractionated heparin (UFH); it has a moderate antidote activity towards low molecular weight heparins (LMWHs) and no activity against Fondaparinux. The Kizhakkedathu and Haynes labs have previously developed a superior antidote to heparins called Universal Heparin Reversal Agent (UHRA). UHRA has two major structural components: a hyperbranched polyglycerol core decorated with positive charges and a brush layer of methoxypolyethylene glycol (mPEG) chains (Figure 1). Compared to protamine, UHRA can be used in a wide range of doses and is highly biocompatible. Also, UHRA can reverse all the clinically used heparins.
In the paper published in Biomacromolecules,1 Dr. Kalathottukaren and team discuss how the rational design of UHRA makes it a better and non-toxic antidote to heparins compared to protamine. The positive charges on the protamine peptide are exposed, which renders it prone to bind nonspecifically to other negatively charged proteins in the blood. Unlike protamine, the positive charges at the core of UHRA are shielded by the mPEG chains that avert non-specific interactions with blood proteins and provide selectivity toward heparins. Using isothermal calorimetry, the group studied the contribution of mPEG chains in UFH-antidote interaction. They observed that naked-UHRA (variant of UHRA lacking mPEG chains) has similar binding profiles to protamine. When they incorporated mPEG chains into UHRA, they found the electrostatic forces of attraction mediated by the positive charges in the core are counteracted by the repulsive steric forces from the mPEG chains. Therefore, for UHRA to interact with a negatively charged molecule, the attractive electrostatic forces have to overcome the repulsive steric forces. This aspect of the molecular design gives the powerful ability to tune the selectivity of UHRA toward heparins by tweaking the number of positive charges and length of mPEG chains.
UHRA is an ensemble of functionally similar molecules with positive charges randomly distributed around the core. The authors argue that, in comparison with protamine, this random distribution of charges helps UHRA to neutralize the unevenly distributed charge of heparins more effectively. Consequently, UHRA can neutralize all clinically used heparins that have varied charge distributions. The authors highlight an interesting point on the biocompatibility of UHRAs: as the mPEG chain length is increased, UHRA becomes more biocompatible. From a therapeutic perspective, this is beneficial, as UHRA could be administered over a wide range of concentrations.
Insights into the molecular basis of therapeutic activity of UHRA show how a strategic design makes it highly specific and biocompatible. This also opens up the possibility of UHRA use in other applications: by fine tuning the number of positive charges or mPEG chain length, UHRA may be used in preventing thrombosis initiated by negatively charged macromolecules, a current clinical challenge.
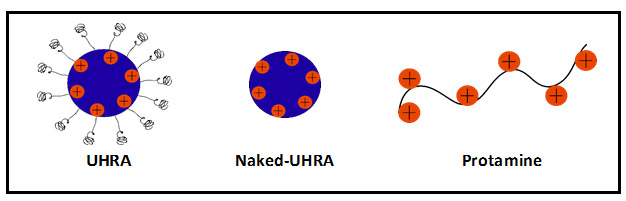
Figure 1: Illustration of UHRAs and Protamine.


