 By Dr. Georgina Butler, Research Associate, Overall Lab
By Dr. Georgina Butler, Research Associate, Overall Lab
Chronic inflammatory and autoimmune diseases, such as arthritis1 and systemic lupus erythematosus2 (SLE), affect many Canadians, and yet the mechanisms behind such conditions are not fully understood and there is no cure available.
Inflammation is crucial for the destruction of infectious agents and injured tissue, and this phase of the immune response is mediated in part, by leukocytes. In particular, controlled polarization between proinflammatory (M1) macrophages3 and healing (M2) macrophages regulates the initiation and resolution of inflammation. The switch to M2 macrophages is vital to avoid the development of chronic inflammatory and autoimmune diseases.
The Overall Lab4 at the UBC Centre for Blood Research is focused on understanding the role of proteases in health and disease. Dr. Antoine Dufour and colleagues have determined a mechanism where precise proteolytic processing controls the M1 to M2 macrophage switch. The research, recently published in Nature Communications5, shows that C-terminal truncation of IFNγ, the cytokine that mediates M1 differentiation, by matrix metalloproteinase-12 (MMP-12) removes the IFNγ receptor binding site, effectively turning off IFNγ-mediated polarization of M1 macrophages.
Using murine models of inflammation, Dufour et al. showed that mice lacking MMP12, or wild-type mice treated with a specific MMP12 inhibitor, had increased IFNγ and M1 macrophage levels. These mice displayed an increased incidence and worse symptoms of arthritis, and in an SLE model, exhibited far greater lymphadenopathy and lupus nephritis. By mining transcriptome datasets from human SLE patients, the group found that in untreated SLE, MMP-12 mRNA was significantly reduced compared with healthy controls, whereas in treated SLE patients, MMP-12 expression was comparable with healthy controls. By immunohistochemistry using region-specific anti-IFNγ antibodies, Dufour et al. showed that human SLE lupus nephritis biopsies had increased intact IFNγ as well as reduced MMP-12 immunostaining.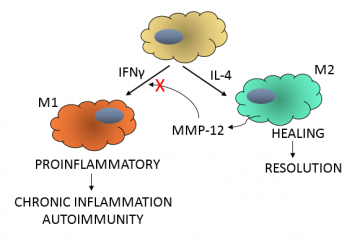
This research highlights a protective role for MMP-12 in dampening inflammation. In a negative feedback mechanism (see figure) MMP-12 secreted from M2 macrophages cleaves the C-terminus from IFNγ. Truncated IFNγ no longer polarizes macrophages to an M1 phenotype, which allows M2 macrophages to resolve the inflammation. Low levels of MMP-12, as seen in SLE patients, would be insufficient to cleave IFNγ and proinflammatory M1 macrophages would persist. Thus therapies directed at augmenting MMP-12 levels might be beneficial in the treatment of chronic inflammatory and autoimmune conditions.
References:


