Written by: Ardin Sacayanan, MASc Student, Laksman Lab 

Edited by: Marie-Soleil Smith, PhD Candidate, Côté Lab
You may have heard of the term “lipid nanoparticles (LNPs)” during the recent coronavirus 2019 (COVID-19) pandemic, but what are they? LNPs are a vessel that can hold pharmaceutical drugs, proteins, or small molecules such as RNA. They effectively deliver loaded components to the correct target and release their contents at the right time (Tenchov et al., 2021). These vessels can be used to target cells such as the hepatocytes that reside in the liver and further upregulate the production of important proteins such as coagulation factors for blood clotting, therapeutic antibodies against diseases like HIV, or inducing fibrosis for liver repair (Witzigmann et al., 2020; Wu & Zern 2000). As this emerging technology continues to evolve, numerous studies have demonstrated optimized procedures to manufacture LNPs with improved efficacy and minimized toxicity (Ferraresso et al., 2022).
The Kastrup group in the Michael Smith Laboratories and Department of Biochemistry & Molecular Biology at UBC compared the effects of two ionizable cations lipids for RNA therapies targeting the liver (Ferraresso et al., 2022). The two molecules used in this comparison study have been pre-approved for clinical trials, namely ALC-0315 and Dlin-MC3-DMA (MC3). ALC-0315 has a similar molecular structure to the lipid used for the COVID-19 vaccines, whereas MC3 is primarily used in small interfering RNA (siRNA) therapies to knockdown a protein produced by hepatocytes which carries and delivers thyroid hormone and vitamin A to different parts of the body.
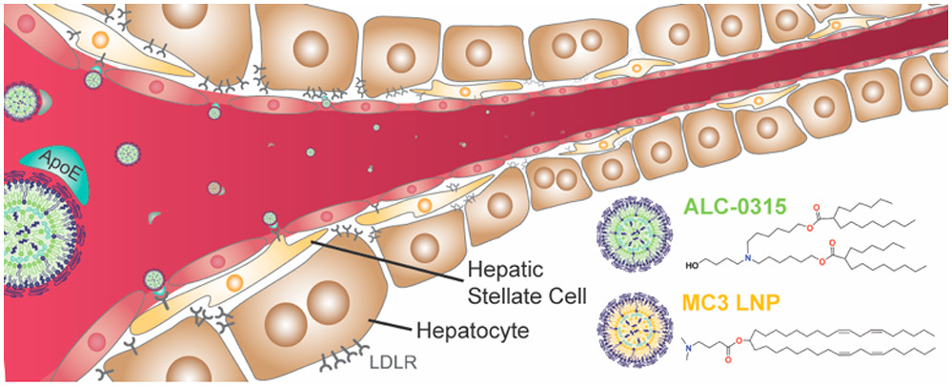
Figure 1. Model of lipid nanoparticle targeting Hepatocytes using the two pre-approved molecules: ALC-0315 (green) and MC3 LNP (yellow)
The Kastrup group compared the efficacy and toxicity of the two molecules by injecting mice with LNP consisting of either ALC-0315 or MC3. Both LNPs were loaded with siRNA ADAMTS13 or coagulation factor VII (FVII). ADAMTS13 targets an enzyme produced by hepatocytes that cleaves von Willebrand factor, which plays a key role in hemostasis by adhering and aggregating platelets, whereas FVII is a central protein in the coagulation cascade (Bartoli et al., 2015). Liver and plasma specimens were isolated from mice to assess the hepatocytes and measure the quantity of siRNA in the serum via western blot and qPCR. Along with the efficiency of each LNP modification, the group was also interested in the hepatoxicity of each molecule. To do this, serum was collected and analyzed via BioAnalytics to evaluate metrics such as liver enzyme levels.
The group demonstrated that ALC-0315 was more potent and efficient in delivering the siRNA for both ADAMTS13 and FVII. However, elevated concentrations of ALC-0315 provided a higher level of alanine aminotransferase and bile acids which can damage liver. Despite this shortcoming, the Kastrup group showed that ALC-0315 are more effective in delivering pharmaceuticals while also showing a proof-of-concept for targeting hepatocytes.
By modifying the structure of LNPs, specific cells such as hepatocytes can be targeted for pharmaceutical therapies. This modification can also be combined with other alterations such as covering the surface of LNPs to consist of ligands that further hone into the target, or by utilizing inert polymers such as polyethylene glycol (PEG) to stealthily sneak through immune cells that would otherwise digest the therapeutics (Tenchov et al., 2021). As the field of lipid nanoparticles continues to grow, researchers and engineers will remain steadfast in improving the efficacy and safety of the vessels as an alternative therapy towards a wide range of diseases. There is so much more to unpack in the world of lipid nanoparticle delivery!
Link to the paper: https://pubs.acs.org/doi/full/10.1021/acs.molpharmaceut.2c00033
Authors: Francesca Ferraresso, Amy W. Strilchuk, Lih Jiin Juang, Lauren G. Poole, James P. Luyendyk, and Christian J. Kastrup
References
- Bartoli, C. R., Kang, J., Restle, D. J., Zhang, D. M., Shabahang, C., Acker, M. A., & Atluri, P. (2015). Inhibition of ADAMTS-13 by doxycycline reduces von willebrand factor degradation during supraphysiological shear stress: Therapeutic implications for left ventricular assist device-associated bleeding. Heart Failure, 3(11), 860-869. https://doi.org/10.1016/j.jchf.2015.06.016
- Ferraresso, F., Strilchuk, A. W., Juang, L. J., Poole, L. G., Luyendyk, J. P., & Kastrup, C. J. (2022). Comparison of DLin-MC3-DMA and ALC-0315 for siRNA delivery to hepatocytes and hepatic stellate cells.Molecular Pharmaceutics, 19(7), 2175-2182. https://doi.org/10.1021/acs.molpharmaceut.2c00033
- Tenchov, R., Bird, R., Curtze, A. E., & Zhou, Q. (2021). Lipid NanoparticlesFrom liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement.ACS Nano, 15(11), 16982-17015. https://doi.org/10.1021/acsnano.1c04996
- Witzigmann, D., Kulkarni, J. A., Leung, J., Chen, S., Cullis, P. R., & van der Meel, R. (2020). Lipid nanoparticle technology for therapeutic gene regulation in the liver.Advanced Drug Delivery Reviews, 159, 344-363. https://doi.org/10.1016/j.addr.2020.06.026
- Wu, J., & Zern, M. A. (2000). Hepatic stellate cells: A target for the treatment of liver fibrosis.Journal of Gastroenterology, 35(9), 665-672. https://doi.org/10.1007/s005350070045


