 By Dr Georgina Butler, Research Associate in the Overall Lab, CBR
By Dr Georgina Butler, Research Associate in the Overall Lab, CBR
Dr Jay Kizhakkedathu’s team, at the Centre for Blood Research, UBC, has designed novel Universal Heparin Reversal Agents (UHRAs) that could be developed for clinical use, but which don’t have the side effects of protamine, the heparin antidote currently in use.
Although essential for normal functioning and hemostatic balance, in some cases blood clotting can pose serious risks and cause heart attacks, stroke, pulmonary embolism or deep vein thrombosis. Heparins, negatively charged chains of varying lengths, are often used as anticoagulants to prevent blot clotting, e.g. during surgery, dialysis, in people with an irregular heartbeat (atrial fibrillation) or as a treatment for clots (thromboembolism). Unfortunately, heparin treatment comes with a serious risk of bleeding, in which case an antidote is required. The only approved antidote for heparin is protamine sulphate, a positively charged molecule. Unfortunately, protamine doesn’t work on all brands of heparin or on shorter heparin chains. It may also have adverse side effects including inflammation, lung injury and independent anticoagulant activity.
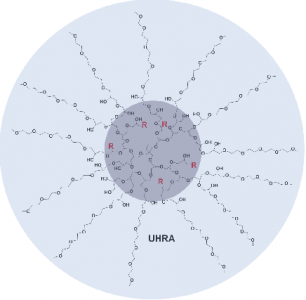
Chemical structure of the Universal Heparin Reversal Agent (UHRA), developed by the Kizhakkedathu lab at the CBR.
Dr Kizhakkedathu’s group set about developing a new generation of heparin reversal agents with less drawbacks than protamine. These non-toxic UHRAs developed by the group in 2014 have a unique structure comprised of a hyperbranched polymer dendritic core decorated with multivalent positively charged binding groups that are shielded by a brush-like layer. The brush prevents non-specific interactions and thus reduces adverse effects.
In their current article published in the journal Blood, Dr Manu Kalathottukaren, a postdoctoral fellow in Dr Kizhakkedathu’s group and the lead author, reports that, unlike protamine, their UHRA does not interact with fibrinogen (a building block for clots), interfere with clot formation, structure or stability, or affect the activity or generation of thrombin (the enzyme that turns fibrinogen into clots). As well as binding to heparin, UHRA also neutralises DNA and polyphosphate, negatively charged factors that can activate blood clotting.
To evaluate the potential for using UHRA as a heparin antidote in patients, the team verified the ability of UHRA to reverse the anticoagulant activity of heparin in mice. Whereas the dose of protamine must be carefully considered, as excess levels impair clotting and cause lung injury, UHRA has no adverse effects on clotting over a wide concentration range. It is also effective at neutralising short chain forms of heparin and maintaining normal clot structure. Importantly, when heparin was neutralised with UHRA, mice were protected against lung hemorrhage and injury that occurred in protamine-treated mice.
“UHRA is a synthetic antidote that can reverse anticoagulation activity of all clinically available heparin-based anticoagulants. The molecule is nontoxic and has a large therapeutic window compared to protamine. The next step is to test the UHRA molecule in a pig, using the cardiopulmonary bypass model.” says Kalathottukaren.
Overall, the improved specificity and reduced toxicity of UHRA is promising for use as a novel heparin antidote. Since the structure of the UHRAs is readily modified, the team can tweak the molecule to improve or alter its binding activities and thus target other detrimental components, such as histone-DNA complexes in sepsis.


