Blood transfusion is a life-saving tool in modern medicine. In spite of the significant advances over the years, the shortage of blood for patients with rare blood groups is still a significant concern. Scientists have been trying to solve this issue for many years with limited success. Utilizing enzymes to create universal blood is a viable option, however, the inefficiency and economic viability of enzymes studied thus far remain an obstacle in bringing the method to clinical relevance.
The difference between the four major blood groups, A, B, AB and O is the sugar structures that decorate the surface of red blood cells. While blood type A and B have the same core sugar structure as blood type O, an additional sugar moiety at the tip of the Type A and B sugar structures trigger the serious life threatening reactions in mismatched patients. The enzymatic removal of these sugars (N-acetylgalactosamine and galactose for type A and B respectively) has the potential to cancel out A, B and O differentiation in vivo (Figure 1). However, current enzymes are inefficient and require amounts large enough to render the approach therapeutically impractical.
A collaborating team including CBR member Jay Kizhakkedathu, and Drs. Stephen Withers and David Kwan from the Department of Chemistry described the development of an improved enzyme that takes us a step closer to achieving universal blood. This outstanding research has been published online in the Journal of the American Chemical Society.
A paper by Kwan et al. revealed that a new enzyme from the family 98 glycoside hydrolase in Streptococcus pneumoniae SP3-BS71 (Sp3GH98) was able to cleave the entire terminal trisaccharide antigenic determinants of both A- and B- antigens from some of the linkages on red blood cell surface glycans. Directed by the enzyme-oligosaccharide crystal structure of the wild-type enzyme, the Withers group engineered a variant with a 170-fold greater efficiency than the parent enzyme (see figure). While this breakthrough significantly advances the state of universal blood, Withers cautions that improvements to the enzymatic removal of N-acetylgalactosamine must be made before clinical studies can begin. However, Withers also states that “Given our success so far, we are optimistic that this will work.”
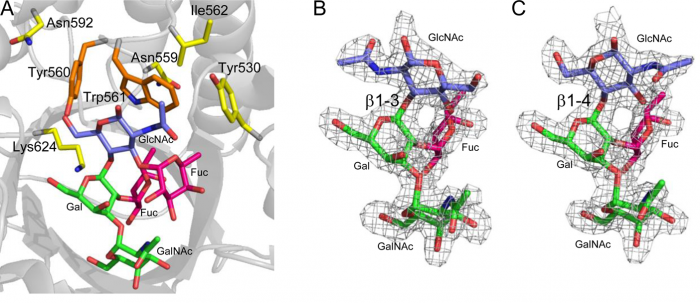
Figure 2: Enzyme-Oligosaccaride complex Structures of Wild-type (A) and Mutants (B,C). Kwan et al. J. Am. Chem. Soc 2015
Research was funded by the Canadian Institute of Health Research (CIHR), Michael Smith Foundation Health Research (MSFHR), Canadian Blood Services (CBS), Health Canada, Canada Foundation for Innovation (CFI), the British Columbia Knowledge Development Fund (BCKDF), and the Canada Research Chairs program. All included quotes were taken from Rajendrani Mukhopadhyay’s article in ASBMB Today.
This publication attracted extensive international media attention, selected links below:
Contributed by Nima Khadem Mohtaram (PDF) and Erika Siren (PhD Student), Kizhakkedathu Lab




