
 Written by: Rhonda Thygesen, MSc Student, Foster Lab (right)
Written by: Rhonda Thygesen, MSc Student, Foster Lab (right)
Edited by: Alexandra Witt, MSc Student, Pryzdial Lab (left)
Blood clots are part of our body’s response to injury, restricting the flow of blood to prevent infection and promote healing. They can, however, be life-threatening and labeled as thrombotic when they block entire veins or arteries1,2. It is estimated that, worldwide, 1 of 4 people die from conditions related to thrombosis, including atherosclerosis, obesity, cancer, and Type 2 diabetes2,3. Understanding the mechanisms that cause thrombosis is an attractive strategy for understanding how we can manage thrombotic disorders.
A number of cells in our body produce tissue factor (TF), a transmembrane glycoprotein4. During injury or cell trauma, TF-bound clotting factor (F) VIIa activates FX and begins the coagulation cascade to restrict bleeding2. TF is also involved in inflammation and cell growth, differentiation, death, and mobility2. A large unknown in TF research is how it is regulated; with findings being of major interest to gain therapeutic insight. This is where researchers in the Conway and Pryzdial labs decided to investigate, in a paper published in the Journal of Thrombosis and Haemostasis.
A large unknown in TF research is how it is regulated; with findings being of major interest to gain therapeutic insight. This is where researchers in the Conway and Pryzdial labs decided to investigate.
Their research took them towards CD248, otherwise known as endosialin, which is a transmembrane glycoprotein just like TF4,5. High levels of CD248 play an important role in encouraging cancer, inflammation (e.g., arthritis, atherosclerosis, obesity), and fibrosis5-7. Its role in coagulation and thrombosis, however, had not yet been evaluated. Similarities between the structure and function of TF and CD248 in cell expression, inflammation, and pathogenesis had our CBR researchers speculating that CD248 may participate with TF to regulate coagulation.
The current findings confirm the association between TF and CD248. When experimenting with mice that lacked CD248, they discovered that those mice missing CD248 experienced less clotting. This association between CD248 and TF would not be coincidental; by culturing cells from wild type and CD248 knockout mice with FX and FVIIa it was discovered that CD248 requires TF to enhance generation of factor Xa. These findings support the role of CD248 as a cofactor in the TF-FVIIa activation of FX.
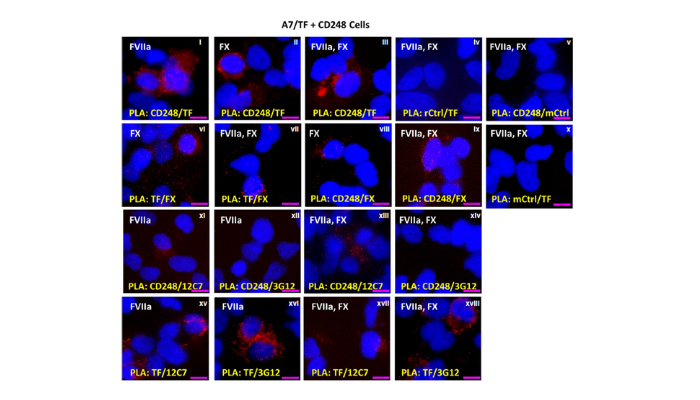
Figure 1: Structure of proximity ligation assay (PLA) using paired antibodies (in yellow) on A7/TF + CD248 cells with mixed pairings of FX and FVIIa (in white). TF and CD248 are in close contact (panels i—iii). FX is near TF and CD248 whether or not FVIIa is present or not (vi—ix).4 In the presence or absence of FX, FVIIa is in close proximity to CD248 with anti-FVII/VIIa antibody 3G12 (xi, xiii), but not with 12C7 (xii, xiv). In the presence of CD248, the close TF-FVIIa interaction was observed when the anti-TF antibody was paired with either 3G12 or 12C7 in the absence of FX (xv, xvi) or in the presence of FX (xvii, xviii). [Figure 7 in-text]
These discoveries not only help us to understand the mechanism by which TF is regulated, but introduce new opportunities for thrombosis therapy. More basic and clinical studies on TF and CD248 are expected to be done, more importantly on their relationship in related disorders and biological systems.
-
Read the journal article published in the Journal of Thrombosis and Haemostasis: “CD248 enhances tissue factor procoagulant function, promoting arterial and venous thrombosis in mouse models”
-
Read about related research on the Canadian Blood Services’s Research.Education.Discovery (R.E.D.) blog: “Investigating envelope viruses: Tissue factor clotting research earns a first-ranked CIHR Project Grant”
References
1 Grover, S. P., & Mackman, N. Intrinsic pathway of coagulation and thrombosis: Insights from animal models. Arterioscler. Thromb. Vasc. 2019;39(3): 331-338. doi:10.1161/ATVBAHA.118.312130
2 Grover, S. P., & Mackman, N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler. Thromb. Vasc. 2018;38(4):709-725. doi:10.1161/ATVBAHA.117.309846
3 Thrombosis. (n.d.). Accessed January 5, 2022. https://www.worldthrombosisday.org/issue/thrombosis/
4 Kapopara, P. R., et al. CD248 enhances tissue factor procoagulant function, promoting arterial and venous thrombosis in mouse models. J. Thromb. Haemost. 2021;19(8):1932-1947. doi: 10.1111/jth.15338
5 Naylor, A. J., et al. The mesenchymal stem cell marker CD248 (endosialin) is a negative regulator of bone formation in mice. Arthritis Rheum. 2012;64(10):3334-3343. doi:10.1002/art.34556
6 Maia, M., et al. CD248 and its cytoplasmic domain: a therapeutic target for arthritis. Arthritis Rheum. 2010;62(12):3595-3606. doi:10.1002/art.27701
7 Simonavicius, N., et al. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod. Pathol. 2008;21(3):308-315. doi:10.1038/modpathol.3801006


