Written by: Mya Bal, Natalia Nayyar & Charlotte Gilmore – Undergraduate Students, McNagny Lab
Edited by: Ahmed Kabil, PhD Student, McNagny Lab & Michael Hughes, Research Associate, McNagny Lab
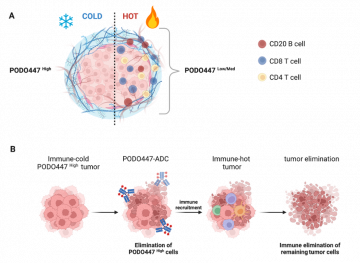
Figure 1. The tumor-specific PODO447 epitope is a new therapeutic target for ovarian cancer treatment. (A) The PODO447 epitope is a new biomarker of ‘cold’ tumors in ovarian cancer. (B) A PODO447 antibody drug conjugate (ADC) may help eliminate tumor cells in chemoresistant and recurrent ovarian cancer by turning ‘cold’ tumors ‘hot’. Figure made with BioRender.com and is a modified version of Fig 6 from Brassard et al, Front. Oncol., 21 December 2023.
Ovarian cancer is a silent killer. With subtle symptoms, it is often detected at an advanced stage. Current gold-standard treatments have major side-effects that diminish patient quality of life. Sadly, despite treatment with surgery and aggressive chemotherapy, the cancer is highly likely to return. More effective and less toxic treatments are urgently needed.
Research by Dr. Julyanne Brassard, recently published in Frontiers in Oncology,[1] highlights a promising new therapeutic target for ovarian cancer. Her study was conducted in the research labs of Prof. Kelly McNagny and Prof. Calvin Roskelley, in collaboration with the BC Cancer Agency (Deeley) and MAPCore. Dr. Brassard found that a tumor-specific form of podocalyxin (PODXL) (the PODO447 epitope) is expressed on a subset of tumor cells in 60% of patients with high-grade serous ovarian cancer (HGSOC). HGSOC is the most prevalent and lethal type of ovarian cancer. Importantly, in HGSOC, the PODO447 epitope is highly expressed in tumors with a ‘cold’ immune profile. Cold tumors lack immune cells that could help eliminate tumor cells. Conversely, ‘hot’ tumors have T and B cells and other inflammatory cells, supplied by the body’s own immune system, to help fight the cancer. Thus, even when treated with chemotherapy, cold tumors are typically associated with the most dismal prognoses. Finding ways to convert ‘cold’ tumors into ‘hot’ tumors would predictably make current treatments more effective.
While normally expressed in some healthy tissue (including blood vessels), PODXL expression on tumor cells is universally linked to poor prognosis because it promotes tumor spread (metastasis). Spread of tumor cells from the site of origin to other organs is most often the cause of death in cancer. Discovery of the PODO447 epitope in HGSOC, particularly in ‘cold’ tumor types, makes it a promising target for treatment since it permits the development of therapies that target hard-to-treat tumors while avoiding healthy tissue. Dr. Brassard’s insights suggest that tumor cells expressing the PODO447 epitope have engaged mechanisms to evade the immune system. She is currently working with collaborators on mapping out the underlying mechanisms of immune evasion in PODO447High HGSOC tumors.
When asked why she thinks cold HGSOC tumors express high levels of the PODO447 epitope, Dr. Brassard said:
When a protein like podocalyxin is expressed on normal cells, there are important processing steps (called glycosylation) that add chains of sugars to ‘decorate’ the mature protein. These glycosylations are important for normal protein function. But, during transformation of normal cells to tumor cells, protein glycosylation is altered. Some of these alterations may provide tumor cells with a survival advantage, help them spread, or help them hide from the immune system. The PODO447 epitope seems to arise from altered glycosylation in tumor cells and it is probably generated by mechanisms linked to the immune evasion characteristics of tumors. This is something I will test in my future research.
In related research[2],[3], the McNagny & Roskelley teams generated a monoclonal antibody (PODO447 mAb) to target tumor-expressed PODXL. When coupled to a potent toxin (ie, an antibody-drug conjugate (ADC)), PODO447-ADC effectively kills human ovarian and pancreatic tumor cells in preclinical mouse models. Brassard is working to optimize the PODO447-ADC prototype and bring this promising new therapy to the clinic.
[1] https://doi.org/10.3389/fonc.2023.1286754


