 By Corrie Belanger, PhD Candidate, Hancock Lab, CBR
By Corrie Belanger, PhD Candidate, Hancock Lab, CBR
Antimicrobial resistance is a serious global threat, causing treatment of even minor infections to become challenging and expensive1. When bacteria are starved or stressed, they can become resistant to antibiotics through activation of certain stress response pathways. This leads to decreased bacterial metabolism, biofilm formation, increased production of virulence factors, and ultimately a bacterial growth state that is more resilient and harder to treat2. In a recent publication in Frontiers in Microbiology, members of the Hancock lab explored the importance of a specific stress response called the stringent response, and its mechanism in infection and virulence of a notoriously drug resistant pathogen, Pseudomonas aeruginosa. Their research indicates that this stringent response plays a large role in skin infections, and that treatment with specific small peptides can target the stringent response to inhibit the adaptive bacterial behaviour, thereby overcoming resistance and virulence in deep skin infections.
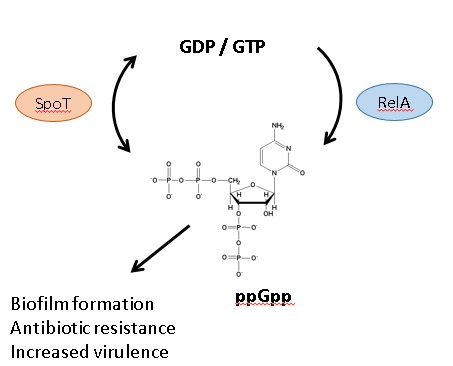
Figure 1. Bacterial stringent response is controlled by enzymes SpoT and RelA which maintain the cellular levels of signaling molecule ppGpp, which ultimately leads to increased biofilm formation, antibiotic resistance, and virulence
Starvation and other stresses that bacteria encounter in a host environment can trigger the stringent response in pathogens after infection. In P. aeruginosa, this process relies on global stress response regulator enzymes RelA and SpoT, which ultimately control the cellular levels of a nucleotide secondary messenger, guanosine tetraphosphate (ppGpp) (Figure 1). High levels of this messenger can alter gene expression and cause the bacteria to switch from susceptible growth states to more drug resistant states such as biofilms2.
Experiments performed by Pletzer et al. used bacterial strains that fluoresced when RelA and SpoT genes were activated to demonstrate that both stringent response genes were expressed during skin wound formation in mice. Bacteria missing these genes were also measured for their ability to lyse red blood cells and for cytotoxicity against human cells. In this case it was shown that losing both stringent response genes at the same time reduced cytotoxicity, and led to a decrease in excreted virulence factors. This supports previous research indicating that the stringent response is important for bacterial virulence and cytotoxicity, and points to the specific enzymes that are involved. It was further demonstrated that targeting the stringent response with small cationic peptides DJK-5 and 1018 had a profound effect on the ability of multiple P. aeruginosa strains to elicit virulence and wound formation.
This research allows scientists to understand more about the mechanisms used by P. aeruginosa during the formation of wound infections and has important implications for the future of clinical treatments. New information in this area can bring to light novel mechanisms for treating clinically relevant infections. The results of this study further support the development of stringent response-targeting substances as alternative methods to antibiotics to fight the wave of devastating drug resistant pathogens we are currently facing.


