 Sporulation is used by some types of bacteria to survive harsh environmental conditions, such as extreme temperatures, chemical disinfection, or periods of nutrient deprivation1. Strains of the spore-forming bacterium Bacillus often cause food-borne illnesses due to cross-contamination; their ability to sporulate allows them to withstand standard sanitation protocols within the food industry2. The process of sporulation begins with unequal cell division of the mother cell, resulting in the formation of a smaller spore engulfed by the membrane of the larger mother cell. Upon formation of the engulfed spore, the mother and spore cells contribute several different proteins to build a continuous channel between them. When completely assembled, this channel is proposed to deliver substrates provided by the mother cell, assisting with the development and metabolism of the spore. Understanding the process of sporulation is imperative to allow further insights into bacterial pathogenesis and evolution of the primitive cell3. Most important, such information will be of value in developing strategies to prevent food-borne illnesses.
Sporulation is used by some types of bacteria to survive harsh environmental conditions, such as extreme temperatures, chemical disinfection, or periods of nutrient deprivation1. Strains of the spore-forming bacterium Bacillus often cause food-borne illnesses due to cross-contamination; their ability to sporulate allows them to withstand standard sanitation protocols within the food industry2. The process of sporulation begins with unequal cell division of the mother cell, resulting in the formation of a smaller spore engulfed by the membrane of the larger mother cell. Upon formation of the engulfed spore, the mother and spore cells contribute several different proteins to build a continuous channel between them. When completely assembled, this channel is proposed to deliver substrates provided by the mother cell, assisting with the development and metabolism of the spore. Understanding the process of sporulation is imperative to allow further insights into bacterial pathogenesis and evolution of the primitive cell3. Most important, such information will be of value in developing strategies to prevent food-borne illnesses.
Natalie Zeytuni and colleagues in the Strynadka Lab at UBC’s Centre for Blood Research have determined the structure of SpoIIIAG, a key protein contributed by the mother cell of the sporulation channel from the Gram-positive bacterium, Bacillus subtilis. SpoIIIAG is proposed to assemble within the inter-membrane space between the spore and mother cell membranes, where it forms a large ring-like structure. This single ring interacts with other proteins to form a continuous channel from the mother cell cytosol to the spore. Zeytuni et al. determined the high-resolution 3D structure of the B. subtilis SpoIIIAG channel using single-particle cryo-electron microscopy (Figure 1). The advancement and fine-tuning of cryo-electron microscopy has progressed the field of structural biology, allowing for structure determination of proteins that are not amenable for X-ray crystallography. This past year, Drs Jacques Dubochet, Joachim Frank, and Richard Henderson were awarded the Nobel Prize in Chemistry for their contribution to the development of this technique.
This work on SpoIIIAG revealed that it is composed of 30 repeating units, representing the highest level of symmetry that has been reported within any protein assembly. Along with the unprecedented size of the SpoIIIAG channel, each identical component of this channel contains a novel protein fold, which Zeytuni et al. have termed the ‘b-triangle motif’ (Figure 1D); this motif is proposed to be the driving force for assembly of the ring complex. Novel protein folds are not commonly reported in present day structural biology, making this discovery of the novel b-triangle motif especially exciting.
Appreciating this important conduit involved in sporulation will aid our understanding of these channels and related biological structures. Using the information acquired here at the Centre for Blood Research for structure-guided drug design could prevent the assembly or function of these protein complexes and related structures in a wide range of pathogenic bacterial species, including Clostridium difficile and Salmonella enterica, thereby improving public health.
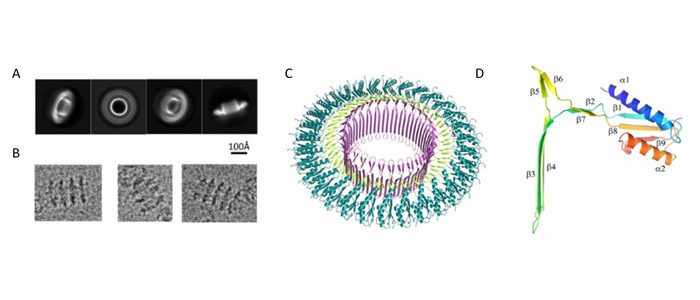
Figure 1: Cryo-electron microscopy structure of SpoIIIAG channel from Bacillus subtilis. (A) Representative 2D class averages obtained from particles picked from cryo-EM micrographs, with sample particles shown in (B). Overview of 3D structure (C); (D) β-triangle motif.
- Hutchison, E. A., Miller, D. A. & Angert, E. R. Sporulation in bacteria: Beyond the standard model. Microbiol. Spectr. 2, TBS-0013-2012 (2014).
- Faille, C. et al. Sporulation of Bacillus spp. within biofilms: A potential source of contamination in food processing environments. Food Microbiol. 40, 64–74 (2014).
- Vollmer, W. Bacterial outer membrane evolution via sporulation? Nat. Chem. Biol. 8, 14–18 (2011).
- Zeytuni, N. et al. Near-atomic resolution cryoelectron microscopy structure of the 30-fold homooligomeric SpoIIIAG channel essential to spore formation in Bacillus subtilis. PNAS Early Edition. 1-9 (2017).


