 By Maria-Elizabeth Baeva, MSc Candidate, Jefferies Lab
By Maria-Elizabeth Baeva, MSc Candidate, Jefferies Lab
When we think about how lymphocytes work, we tend to think that antigen presentation and signaling are the be-all and end-all of immune system activation and function. However, there are many carefully organized and necessary steps that must occur in order to have a proper immune response. One of these crucial elements is the calcium (Ca2+) channel. In lymphocytes, Ca2+ channels are responsible for mediating not only activation, but also homeostasis, maturation, and apoptosis. This study is the first to demonstrate a link between a mutation in the voltage-dependent L-type Ca2+ channel CaV1.4 and lymphocyte dysfunction and exhaustion.
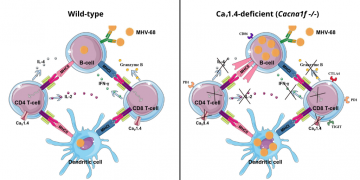
Comparison of immune cell profiles between wild-type (left) and CaV1.4-deficient (right) mice. In CaV1.4-deficient mice, there are memory T cell phenotype, an increase of PD-1 levels restricted to the CD4 T-cell, increased CTLA-4 levels in T cells, and decreased Ca2+ flux in T cells.
Immune exhaustion has recently come to some mainstream attention thanks to Drs. James P. Allison of the University of Texas MD Anderson Cancer Center and Tasuku Honjo of Kyoto University sharing the 2018 Nobel Prize in Physiology or Medicine. They received the prize for their respective discoveries in cancer therapy by inhibiting two of the most researched negative immune regulators: CTLA-4 and PD-1. These receptors are present on lymphocytes as inhibitory markers, meaning they prevent the immune system from over-reacting and causing an autoimmune disorder. However, chronic exposure to pathogens can lead to immune system exhaustion, or a constant activation of cells, leading to a constant presence of these markers and the cells being unable to clear pathogens effectively.
Fenninger et al. were able to link mutations in CaV1.4 with lymphocyte dysfunction, expression of inhibitory markers, and immune exhaustion by looking at cell types and frequencies in a mouse model with the CaV1.4 gene (Cacna1f) knocked out. By analyzing spleens of mice using flow cytometry, they were able to determine that while a disruption in the CaV1.4 gene decreased overall CD8+ T cell frequency, there was an increased frequency of both CD8+ and CD4+ effector memory T cells (which are responsible for rapidly dealing with a previously recognized pathogen) and indications of chronically activated B cells. Many of the lymphocyte subtypes also had an upregulation of the inhibitory factors PD-1 and/or CTLA-4. Interestingly, when they tested Ca2+ flow, although the T cells had a decreased Ca2+ flux, it appears that the B cells had some compensatory mechanisms, since their Ca2+ flux was unaffected.
To characterize the functioning of these lymphocytes in vivo, CaV1.4 KO mice were infected with a murine version of a chronic Epstein-Barr virus infection. This mimics a situation wherein patients with a primary immune disorder are infected with a common virus. The infected, mutated mice had higher CD4+ T effector cell and lower CD8+ naïve T cell counts compared to wild-type infected mice. What’s more, the central memory T cells, which are responsible for remembering and mounting a stronger response to the virus should it reappear, in infected CaV1.4 KO mice demonstrated a greater frequency of PD-1+ cells than the central memory T cells of infected wild-type mice. This suggests that these lymphocytes are unable to clear the infection and thus the infection will remain ongoing.
This study effectively demonstrated how a combination of genetic and environmental factors can trigger immune dysfunction. To read more about it, check out the Science in the City article, which interviewed the final author of the paper, Dr. Wilf Jefferies.
Citations:
Fenninger F, Han J, Stanwood SR, et al. Mutation of an L-Type Calcium Channel Gene Leads to T Lymphocyte Dysfunction. Front Immunol. 2019;10:2473. Published 2019 Oct 29. doi:10.3389/fimmu.2019.02473


