 By Tseday Zewdu Tegegn, PhD Student, Pryzdial Lab
By Tseday Zewdu Tegegn, PhD Student, Pryzdial Lab
Cardio- and cerebro-vascular diseases remain the leading causes of death worldwide. These pathologies are strongly linked to a skewed balance between the two physiological mechanisms, clot-formation and dissolution. These processes must be carefully controlled to maintain healthy vessels and blood circulation. When blood clots are formed to stop excess bleeding, they keep us alive! However, when the clot formation process fails to stop and too much of it persists, there can be fatal consequences such as heart attacks and strokes.
Many factors contribute to clot-formation and persistence. Often overlooked agents like viruses have a track record of association with the disruption of blood clotting mechanisms. Thus, they can contribute to cardiovascular diseases, including myocardial infarction, atherosclerosis, thrombosis, and bleeding. As examples, herpes simplex virus (HSV), human immunodeficiency virus (HIV), hepatitis C virus, Ebola virus, and dengue virus infections have been reported in multiple cardiovascular pathologies. Viral infections have been major global health threat throughout history of mankind. Like no other time, this fact is apparent and pressing as the SARS-CoV-2 pandemic limits our daily function as a society.
Viruses often evolve to infect diverse hosts by using multiple ways to help them evade defence mechanisms that challenge their persistence. One method, particularly for enveloped viruses, is acquiring host membrane proteins in their outer structure. These proteins can have different functions including roles in blood coagulation. Within the blood vessels, the goal of these viruses is to initiate the clot-formation process in order to stimulate host cells via surface molecules such as protease activated receptors (PARs). Subsequently, the viruses prompt a cascade of message transmitted into the host cells that prime and enhance virus infectivity, ultimately helping the virus thrive.
The Pryzdial laboratory, at the Centre for Blood Research at the University of British Columbia, has been investigating mechanisms of clot-formation enhancement by enveloped viruses for over 20 years. Previously, using HSV-1 as a model for enveloped viruses, they demonstrated its contribution to clot-formation and dissolution (see studies here and here). HSV-1 is known to cause oral herpes. As an enveloped virus, HSV-1 provides host-derived surface factors such as anionic phospholipids (aPL) (an essential molecule for the assembly of factors preceding clot-formation/dissolution) and tissue factor (TF) (an initiator of clot-formation).
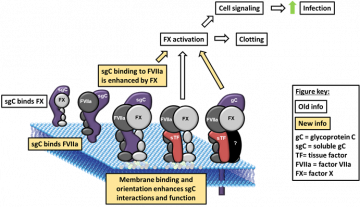
HSV-1 promotes blood clotting not only by incorporating host-derived clotting factors like aPL and TF but by using its own multifunctional molecule, glycoprotein C (gC). gC is primarily involved in several important viral functions. gC initiates the first step of the viral life cycle by aiding its docking on extracellular molecules known as glycosaminoglycans, which are present on a broad spectrum of host cells. When it comes to its involvement in clot-formation, gC provides additional surface binding sites to factor X (FX), crucial to the generation of activated FX (FXa), through a factor FVIIa-dependent mechanism.
To give you a bit of mechanistic background, FXa generation is a key step towards the clot-formation and stabilization. This process involves thrombin generation and conversion of fibrinogen into fibrin. Interestingly, though the activity is reduced approximately 1000-fold, gC contributes to FXa generation even when not tethered to the virus surface. In addition, when TF is present on HSV-1, increased infection was shown in blood vessel endothelial cells by priming them through PARs receptors. More specifically, the effect through PAR-2 was inhibitable using anti-TF antibody in cells pretreated with FXa, FVIIa and plasmin (clot-dissolution factor).
In their newest publication authored by Dr. Bryan H. Lin, also using HSV-1, the Pryzdial lab discussed their further investigation on the influence of gC on FVIIa-dependent FX activation. More specifically, the role of host-derived aPL and interaction with FVIIa and FX to the contribution of gC to the FXa generating complex. To dissect this complex, the team utilized biochemical assays and electron microscope imaging techniques to confirm the simultaneous expression of gC, TF and aPL on a single HSV-1 particle and the requirement of viral TF availability to enhance FXa generation by gC.
Interestingly, the study implies that although gC parallels the function of host-derived TF, it may involve other HSV-1 envelope constituents worth investigating in future studies. Furthermore, gC mimics TF cofactor function being able to bind the protease FVIIa and substrate FX enhancing the cofactor/protease affinity. This ensures the generation of localized product for cell stimulation through the neighbouring PARs.
In summary, the Pryzdial lab’s new investigation has future implications on mitigating virus-induced cardiovascular pathogenesis through pan-specific antivirals targeting common host factors such as TF and PARs. Potentially, structural and functional knowledge may help develop gC-inspired therapeutics to enhance blood clotting in bleeding patients without eliciting an immune response. Overall, advancement in understanding of how “heartfelt viruses” contribute to clot-formation and disrupt homeostasis is essential and timely as researchers scramble to gather wisdom in pursuit of finding therapeutic solutions to COVID-19 which also happens to be an enveloped virus.
Do not forget to check out the full article for more scientific information! Stay safe!
Reference:
Coagulation factor VIIa binds to herpes simplex virus 1-encoded glycoprotein C forming a factor X-enhanced tenase complex oriented on membranes. Lin BH, Sutherland MR, Rosell FI, Morrissey JH, Pryzdial ELG. J Thromb Haemost. 2020 Mar 7.
Virus envelope tissue factor promotes infection in mice. Sutherland MR, Simon AY, Shanina I, Horwitz MS, Ruf W, Pryzdial ELG. J Thromb Haemost. 2019 Mar;17(3):482-491.
Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease-activated receptor 2 cofactors that enhance infection. Sutherland MR, Ruf W, Pryzdial EL. Blood. 2012 Apr 12;119(15):3638-45.


