By Solmaz Sobhanifar, PDF in Strynadka Lab
By Solmaz Sobhanifar, PDF in Strynadka Lab
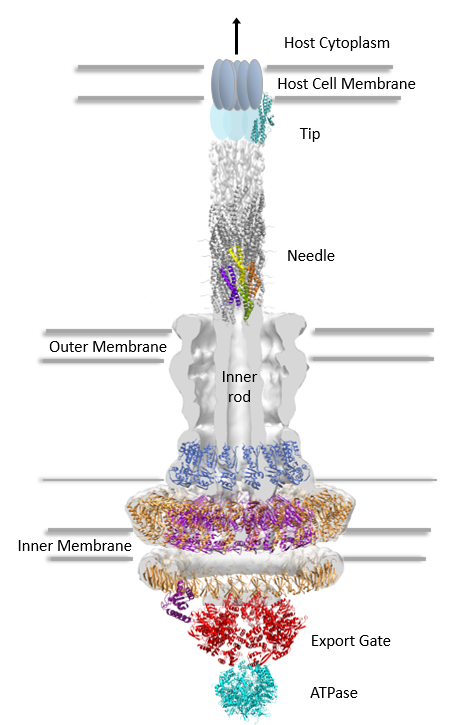
It is fascinating to behold the ingenuity by which certain bacteria achieve infection in their host. One clear example is the enteropathogenic Escherichia coli, which uses a molecular syringe and needle complex (also known as the Type III secretion system) to inject its virulence proteins into the cytoplasm of host intestinal epithelial cells. The Type III (T3SS) secretion system (one of six secretion systems present in Gram-negative bacteria) is composed of over 30 different proteins. In order to assemble, the T3SS must in addition to the inner and outer cellular membranes, traverse a tight mesh-like barrier, the peptidoglycan (PG) layer, present in bacterial cell walls. In order to make room for the needle complex assembly, the T3SS requires a dedicated lytic enzyme to locally cleave the PG. In E. coli, the lytic enzyme EtgA assumes this role.
In a recent paper published in the journal of Biological Chemistry, Brianne Burkinshaw, a PhD candidate in the Strynadka laboratory at CBR, was successful in solving the crystal structure of EtgA and made the interesting observation that this enzyme shares features of both lytic transglycosylases and hen egg-white lysozymes. As these two classes of enzymes produce distinctly different PG cleavage products, it remains to be seen whether the dual characteristics of EtgA simply reflect its evolutionary origins or somehow play a role in T3SS assembly, considering that many of the T3SS components interact with PG.
The catalytic activities of PG-lytic enzymes are tightly regulated in order to prevent superfluous or erroneous PG clearance. To this purpose, the authors also confirmed the interaction of EtgA with a T3SS inner rod component, EscI, suggesting spatial restriction of EtgA, and moreover, demonstrated an eight-fold enhanced EtgA activity in the presence of EscI. The authors hypothesize that low EtgA activity in absence of the T3SS rod EscI may be a mechanism by which the system can prevent uncontrolled lysis.
Given that the T3SS is essential in the pathogenesis of many Gram-negative bacteria, unveiling component structures and mechanisms involved in T3SS assembly, such as PG lysis, contributes to drug designing efforts to target this intricate system. EtgA, being one of the few catalytic components of the T3SS, is an attractive pharmaceutical target, as inhibition of its activity will “disarm” E. coli and diminish its virulence without creating a strong selective pressure that often results in drug resistance.