 By Dr. Stefanie Novakowski, Kastrup Lab
By Dr. Stefanie Novakowski, Kastrup Lab
Alzheimer’s disease is a devastating neurological disorder with no known cure. Characterized by gradual cognitive decline, its effects are felt throughout Canada. Over half a million Canadians are living with dementia, and Alzheimer’s disease is a major contributor to this number.1 There are several links between Alzheimer’s disease pathology and blood clotting, however the biochemical pathways connecting these two processes are poorly defined. In a step towards clarifying this relationship, members of Kastrup and Jefferies Labs at the Centre of Blood Research recently characterized the role of the essential clotting protein, coagulation factor XIII (FXIII), in the development of harmful beta amyloid deposits found in Alzheimer’s disease.2
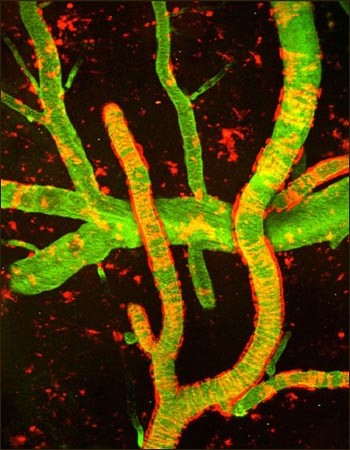
Multiphoton image of cerebral amyloid angiopathy (red) on blood vessels (green) in Alzheimer’s disease mouse model. From cover of Neuron, 2010 Jun 10. Image credit: Cortes-Canteli, M. et al. 2010, 66(5). 695–709. doi: [10.1016/j.neuron.2010.05.014]
In the recent publication, W. Hur and N. Mazinani show for the first time that beta amyloid is a substrate for FXIIIa. FXIIIa obtained from either plasma and platelets can link beta amyloid proteins together to form large, multi-protein aggregates. They also found the activity of FXIIIa and extent of beta amyloid linkage differed for variants of beta amyloid. As these variants are associated with different probabilities of developing CAA, this suggests a possible role for FXIIIa in CAA and possibly Alzheimer’s disease pathology. Unexpectedly, FXIIIa could also link beta amyloid to fibrin, leading to stiffer clots. This leads to the question, is beta amyloid a normal component of blood clots in the brain, or could its incorporation into clots contribute to CAA?
Whether FXIIIa acts on beta amyloid in physiological settings has yet to be determined. To establish this, experiments using animal models will be required. Furthermore, it is still unclear whether FXIIIa contributes to the pathology of CAA and Alzheimer’s disease. However, the work described here helps strengthen the bridge between Alzheimer’s disease and blood clotting. Characterizing these connections is the first step in understanding what causes Alzheimer’s disease, and eventually finding a cure.
- Alzheimer’s Society Canada (2018) Canada’s national dementia strategy: latest information and statistics. Retrieved from http://alzheimer.ca/en/Home/Get-involved/Advocacy/Latest-info-stats
- Hur, W.S., Mazinani, N., et al. Coagulation factor XIIIa crosslinks amyloid b into dimers and oligomers and to blood proteins. J. Biol. Chem. 2018. 2018 Nov 8. pii: jbc.RA118.005352. doi: 10.1074/jbc.RA118.005352. [Epub ahead of print]
- Love, S., Miners, S., Palmer, J., Chalmers, K., and Kehoe, P. (2009) Insights into the pathogenesis and pathogenicity of cerebral amyloid angiopathy. Frontiers in Bioscience. 14, 4778-4792.


