
By Rolinda Carter, PhD Student in the Pryzdial Lab
Heart attack and stroke are leading causes of death worldwide and are due to excess clot blocking the normal flow of blood. To treat these clots, tissue plasminogen activator (tPA), the key physiological enzyme that initiates clot dissolution, was developed into a therapeutic drug and is co-administered with a blood thinner. The ultimate purpose of tPA is to generate plasmin, the enzyme directly responsible for breaking down the clot. While having saved many lives, the use of tPA in the clinic complicated by side-effects including an increased risk of bleeding due to systemic plasmin generation and re-appearance of abnormal clots. To date, advances at finding a safer and more effective therapeutic to replace tPA have been unsuccessful.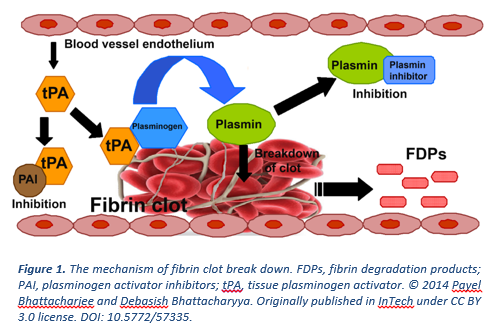
By thinking “out of the box”, the Pryzdial lab at the Centre for Blood Research previously showed that clotting factor Xa (FXa) is modified by plasmin-cleavage to acquire clot-dissolving properties. This was the first time that FXa was shown to have this schitzophrenic role in controlling blood flow. Capitalizing on this finding, in the latest Journal of Thrombosis and Haemostasis publication, Dr. Pryzdial and team collaborated with Dr. William Sheffield at McMaster University to generate and test a chemically modified form of FXa called Xai-K. This new form of FXa was designed to stabilize C-terminal lysine exposure, which facilitates the assembly of tPA and its substrate plasminogen, and thus increases generation of plasmin. Since Xai-K has to be associated to the clot or pro-coagulant surfaces to exhibit this function, localized instead of systemic plasmin generation is achieved.
Through a number of in vitro and in vivo experiments, the authors found that Xai-K was not only able to dissolve clots at a faster rate than tPA-based therapeutics (Alteplase and Tenectaplase), but it also functions as a blood thinner through competition with normal FXa. Hence, an alternative to the current treatment used has been identified.
“It was amazing to see how fast clots cleared when Xai-K was given to a mouse compared to today’s most common therapeutic” – commented Scott Meixner, the research assistant in the Pryzdial lab who generates and characterizes Xai-K.
In addition to using Xai-K alone, the team also showed that Xai-K could be administered in combination with a 34-fold lower dose of the tPA analogue, Tenectaplase, to restore blood flow. The latter is a substantial finding as decreasing the dose of tPA will ultimately decrease the bleeding risk in patients and still enable clearance of highly resistant clots.
“Two decades of major clinical trials aimed at improving the safety of clot-dissolving drugs have been disappointing. Xai-K marks a new strategy to dissolve clots based on a novel biochemical pathway our lab discovered. We’re excited that its preclinical safety and efficacy profiles suggest promising advances are finally on their way” – said Dr. Ed Pryzdial, the principal investigator and first author of the paper. Further studies are currently in progress as the lab seeks to take this technology away from the bench and closer to the bedside.


